Non-mining methane production methods
 Fot.
Fot.
SNG (synthetic natural gas) can be produced by one of two chemical reactions: of hydrogen with carbon monoxide or hydrogen with carbon dioxide, according to the following formulas:
CO + 3 H2 => CH4 + H2O
CO2 + 4 H2 => CH4 + 2 H2O
Methanation is a highly exothermic process; the heat of the reaction requires efficient dissipation from the reactor system. Depending on the reaction components, the resulting gas is called SNG or biomethane (in the latter case, the gas is produced from biomass).
There are several concepts for sourcing the reaction components. They can include biogas from biogasification plants, synthesis gas from gasification processes, and carbon dioxide from the combustion of coal at power plants.
Hydrogen is synthesized in RES-powered electrolysis cells. Oxygen is a by-product of the synthesis; it can be sold or used for gasification processes in power engineering or as a fuel for pure-oxygen combustion, a method which simplifies the capturing of CO2 from power generation installations.
There are several concepts for sourcing the reaction components. They can include biogas from biogasification plants, synthesis gas from gasification processes, and carbon dioxide from the combustion of coal at power plants.
Hydrogen is synthesized in RES-powered electrolysis cells. Oxygen is a by-product of the synthesis; it can be sold or used for gasification processes in power engineering or as a fuel for pure-oxygen combustion, a method which simplifies the capturing of CO2 from power generation installations.
Biological methanation (also known as bio-methanation) is an exothermic reaction supported by living microorganisms. The reaction formula is:
CO2 + 4H2 -> CH4 + 2H2O
Bio-methanation requires a supply of carbon dioxide and hydrogen for a reactor in which anaerobic fermentation occurs. Biological technologies are now developed to enable manufacturing of other products, including bioplastics, bioalcohols, bio-Diesel fuel, proteins, and more. There is genetic engineering research in progress to improve the efficiency of CO2 usage by bacterial cultures [1].
CO2 + 4H2 -> CH4 + 2H2O
Bio-methanation requires a supply of carbon dioxide and hydrogen for a reactor in which anaerobic fermentation occurs. Biological technologies are now developed to enable manufacturing of other products, including bioplastics, bioalcohols, bio-Diesel fuel, proteins, and more. There is genetic engineering research in progress to improve the efficiency of CO2 usage by bacterial cultures [1].
Bio-methanation is performed in reactors. A bio-methanation reactor is a vessel which houses living cultures of bacteria, called “biocatalysts” in these applications. The process flowchart of a bio-methanation system integrated in a farming biogas plant is shown in the figure to the side.
The main component of the process is the bio-methanation reactor, while the greatest challenge to the research into the technology is the improvement of unit efficiency or unit yield. Stirred-tank reactors are the most widespread type of bio-methanation reactors. The bio-methanation reactors for thermo-chemical conversion of CO2 and H2 into CH4 have a considerably high service volume.
The main component of the process is the bio-methanation reactor, while the greatest challenge to the research into the technology is the improvement of unit efficiency or unit yield. Stirred-tank reactors are the most widespread type of bio-methanation reactors. The bio-methanation reactors for thermo-chemical conversion of CO2 and H2 into CH4 have a considerably high service volume.
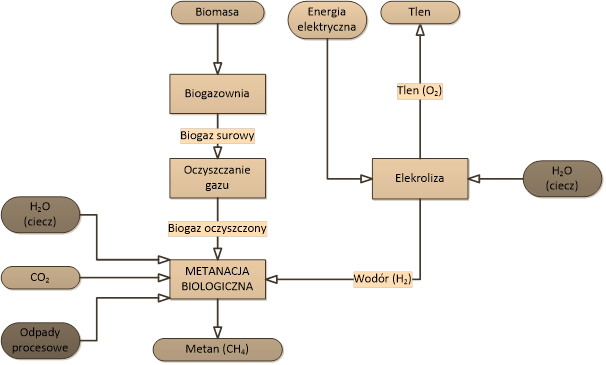
Scheme of the methane production process using biological methanation
The second most important unit of the bio-methanation process is a water electrolysis module (which is usually based on alkaline electrolysis cells). Alkaline electrolysis cells are commercially available, although work on their development and improvement still continues. The drawbacks of alkaline electrolysis cells include difficult operation under variable loads and with electrical power supply interruptions, and long startup time (between 30 to 60 minutes).
The performance of bio-methanation plants based on alkaline electrolysis cells suffers when it relies on power from wind farms or photovoltaic panels, given the uncontrolled conditions of supply from these sources of electricity. Alkaline electrolysis cells require an electrolyte which is extremely corrosive; this means high costs of servicing and maintenance of the bio-methanation plant. The alkaline electrolysis cells require an overhaul every 7 to 12 years. Their estimated service life is 30 years.
The performance of bio-methanation plants based on alkaline electrolysis cells suffers when it relies on power from wind farms or photovoltaic panels, given the uncontrolled conditions of supply from these sources of electricity. Alkaline electrolysis cells require an electrolyte which is extremely corrosive; this means high costs of servicing and maintenance of the bio-methanation plant. The alkaline electrolysis cells require an overhaul every 7 to 12 years. Their estimated service life is 30 years.
The process substrates are continuously supplied to the bio-methanation reactor. According to the reaction formula, the volume ratio of CO2 and H2 is 4 to 1. The bio-methanation reactor is fed with water which is mineralised specifically to provide an optimum environment to facilitate the growth of the bacterial cultures.
The experimentally proven H2 conversion ratio is between 97% and 99%. One of the main beneficial characteristics of the reactors tested so far is a high tolerance to contamination of the process input substrates. Bio-methanation requires a feed of carbon dioxide (CO2) or biogas, or the output gas from thermal biomass gasification. The high tolerance to contaminants makes different stock gases viable for the process. Bio-methanation also requires an input of electrical power.
The experimentally proven H2 conversion ratio is between 97% and 99%. One of the main beneficial characteristics of the reactors tested so far is a high tolerance to contamination of the process input substrates. Bio-methanation requires a feed of carbon dioxide (CO2) or biogas, or the output gas from thermal biomass gasification. The high tolerance to contaminants makes different stock gases viable for the process. Bio-methanation also requires an input of electrical power.
Crude SNG must be adjusted to the transmission pipeline specifications by drying and removal of potential contaminants which could cause poor performance of gas distribution systems.
There are two bio-methanation R&D projects underway in Europe. These are:
· Biopower2gas (http://www.biopower2gas.de)
· P2G_Biocat (http://biocat-project.com/about-the-project/technology-components)
The Biopower2gas project is a bio-methanation R&D installation established in Allendorf (Germany). It uses a PEM (proton exchange membrane) electrolysis cell rated at 300 kW of power and a H2 (hydrogen gas) output of 60 Nm³/h. The installation features a 5 m³ biological reactor. It was constructed as part of the Biopower2gas project.
There are two bio-methanation R&D projects underway in Europe. These are:
· Biopower2gas (http://www.biopower2gas.de)
· P2G_Biocat (http://biocat-project.com/about-the-project/technology-components)
The Biopower2gas project is a bio-methanation R&D installation established in Allendorf (Germany). It uses a PEM (proton exchange membrane) electrolysis cell rated at 300 kW of power and a H2 (hydrogen gas) output of 60 Nm³/h. The installation features a 5 m³ biological reactor. It was constructed as part of the Biopower2gas project.
The P2G_Biocat project features a pilot bio-methanation plant at Spildevandscenter Avedøre (Denmark) with a 1 MW electrolysis cell. The project is planned to feature a 10-megawatt electrolysis cell plant in Hungary.
Chemical methanation is similar to bio-methanation, but it requires a dedicated catalyst (which is usually made of nickel). Other gas components, including ethylene and BTX (benzene, toluene and xylenes) can be converted into methane if a suitable catalyst material is used. An advantage of chemical methanation over bio-methanation is a lower footprint of the process equipment, with a simpler startup and system performance control.
Chemical methanation is similar to bio-methanation, but it requires a dedicated catalyst (which is usually made of nickel). Other gas components, including ethylene and BTX (benzene, toluene and xylenes) can be converted into methane if a suitable catalyst material is used. An advantage of chemical methanation over bio-methanation is a lower footprint of the process equipment, with a simpler startup and system performance control.
Bio-methanation and chemical methanation are technologies currently being developed by power engineering corporations in Poland and abroad. The chemical methanation projects of note included CO2-SNG in Poland and GAYA in France.
Microalgae are likely to become a fuel for future synthesis of bio-methane.
[1] Górski W., Jabłońska M.M.: Eter dimetylowy – uniwersalne, ekologiczne paliwo XXI wieku. NAFTA-GAZ, wrzesień 2012.
Microalgae are likely to become a fuel for future synthesis of bio-methane.
[1] Górski W., Jabłońska M.M.: Eter dimetylowy – uniwersalne, ekologiczne paliwo XXI wieku. NAFTA-GAZ, wrzesień 2012.